Who We Are

Wuhan Huamei Biotech Co.,LTD.,located in "Optics Valley of China" founded in 2007, is a high-tech enterprises of independently researching& producting and selling protein, antibody, diagnostic reagent raw materials, scientific research kits and other products. Relying on experts and technical teams from Wuhan University, Wuhan Institute of Virology, Chinese Academy of Sciences, Huazhong University of Science and Technology, Huazhong Agricultural University and other universities and research institutes, the company has provided related products and customized technical services for well-known domestic and foreign manufacturers of diagnostic reagents, pharmaceutical R&D companies, universities, enterprises and research institutes. Industrial Raw Materials Division is an independent production line of high-quality biological raw materials of Wuhan Huamai Biotech Co.,LTD., currently focusing on the research and development, production and sales of raw materials used in in vitro diagnostic reagents and other fields.
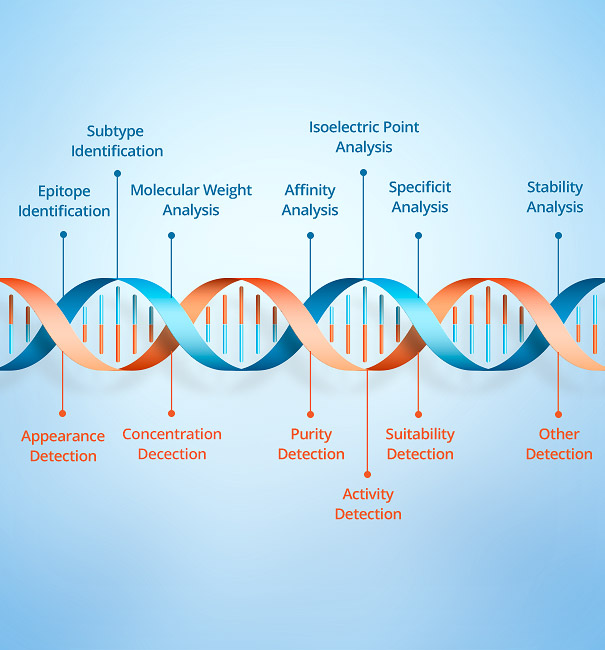
Industrial Raw Materials Division can provide the majority of in vitro diagnostic reagent manufacturers with high quality monoclonal antibodies, polyclonal antibodies, diagnostic proteins, biochemical enzymes, molecular biology and general raw materials, at the same time can provide customers with OEM reagents and professional customized services. The products are comprehensively cover inflammation markers, cardiac markers, tumor markers, liver and kidney function, thyrohormone, autoimmune diseases, respiratory pathogens, animal infectious diseases and other fields; Widely used in CLIA,TRF-LFIA,ELISA,LETIA and other platforms.
Company Culture
Development History

- 2007
Huamei Biotech Co., LTD. was founded in Wuhan.
- 2008
The technology platform of recombinant protein and enzyme-linked immunity established.
- 2009
The technology platforms of monoclonal antibody (hybridoma) and polyclonal antibody established; ISO 9001 certification acquired.
- 2010
The technology platform of small molecule synthesis and complete antigen modification, latex immunity turbidimetric technology platform and protein basic performance analysis and detection platform established.
- 2011
The analysis platform of Enzyme engineering technology platform and clinical biochemistry established.
- 2012
ISO 9001 certification acquired again.
- 2013
The project management standardization system implemented; Monoclonal antibody production line (hybridoma) Improved.
- 2014
Improving the recombinant protein production line; Establishing the chemiluminescence immunoassay platform.
- 2015
CUSABIO · CUSAg Diagnostic Raw Materials Division founded; The colloidal gold analysis platform established.
- 2016
Postdoctoral innovation practice base approved; the quality function into the TQM era improved.
- 2017
The immunofluorescence chromatography platform and phage display platform established.
- 2018
The Nobel Prize workstation established.
- 2019
A research and development team of molecular diagnostic raw materials for molecular enzyme products established.
- 2020
P2 laboratory qualification acquired; A single B cell antibody technology platform established.
- 2020
The lyophilized protein production platform put into use and played a great role in the production of proteinase K products.
- 2021
1000㎡GMP clean workshop put into use; The Molecular Diagnostics Centre has been relocated and upgraded.
- 2022
Academician workstation established; ISO 9001 &ISO 13485 dual-system certification preparation period;National-level specialized and special new "little giant" enterprises.
- 2023
Customs AEO advanced certification; CUSABIO·CUSAg Diagnostic Raw Materials Division changed to Wuhan Huamei Biotech Co.,LTD.◎Industrial Raw Materials Division
Core Competition
Quality Assurance
Company Honor









Global Market
Service coverage 30+ countries and regions
Our products have been recognized by 1000+ customers at home and abroad

Wu Han