Analysis of Inflammation Marker---Serum amyloid A1 (SAA1)
Views:1103 Add time:2018-10-26 13:16:25
Serum amyloid A1 (SAA1) is a protein that in humans is encoded by the SAA1 gene. SAA1 is a major acute-phase protein mainly produced by hepatocytes in response to infection, tissue injury and malignancy. When released into blood circulation, SAA1 is present as an apolipoprotein associated with high-density lipoprotein (HDL). SAA1 is a major precursor of amyloid A (AA), the deposit of which leads to inflammatory amyloidosis.
Serum amyloid A (SAA) and C reactive protein (CRP) are the most sensitive acute phase proteins in plasma. Plasma concentrations of these proteins increase up to 1000-fold of their physiological levels in response to inflammation. In plasma both SAA and CRP are believed to be derived mostly from the liver. Hepatocytes secrete these proteins by the induction of inflammation related cytokines such as tumour necrosis factor α, interleukin 1, and interleukin 6. Although some differences in cytokine regulation by hepatocytes has been noted between SAA and CRP, clinical observations suggest that plasma concentrations of these two proteins are highly correlated with each other in most inflammatory diseases.
Anti-HumanSAA1 Monoclonal Antibodies
Anti-SAA1monoclonal antibody has been developed for five years, whichcould be used indevelopment of immunoassays for human serum amyloid A1. All the antibodies wereevaluated in CUSAg in-house colloidal gold immunochromatogragphic assay and latexenhanced immunoturbidimetric assay, respectively.
|
Properties |
Specification |
|
Target species |
Human |
|
Host animal |
Mice Balb/c |
|
Cell line used for fusion |
Sp2/0 |
|
Immunogen |
Humanserum amyloid A1 |
|
Purification method |
Protein G affinity chromatography |
|
Purity |
>90% (SDS-PAGE) |
|
Presentation |
MAb solution in NaCl with 15 mM NaN3 (pH 7.2) |
|
Application |
LFIA, LETIA |
|
Catalog Number |
CSB-DA118BmN② CSB-DA118BmN⑤ |
Calibration Curve
LFIA platform
A set of SAA1calibrators with the concentration of 0, 0.5, 2, 10, 20, 50, 100 and 200mg/L was detected on CUSAg LFIA platform using two anti-SAA1 monoclonal antibodies. The capture antibody was stripped on the nitrocellulose membrane,and the detection antibody wasconjugated to colloidal gold.The best selected MAb combination for the development of semi-quantitative human SAA1immunoassaysis (capture-detection):
CSB-DA118BmN②- CSB-DA118BmN⑤
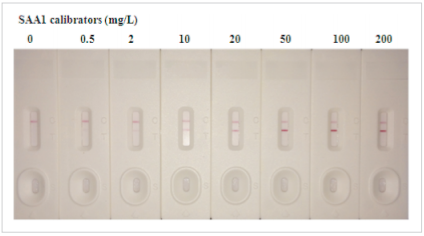
Fig.1Semi-quantitativedetection of SAA1 protein in colloidal gold immunochromatogragphic assay
LETIA platform
SAA1 antigens specifically react with anti-human SAA1 monoclonal antibodies which were precoated onlatex beads, resulting in agglutination and increase in turbidity. The calibration curve was fitted according to the relationship between absorbance values and SAA1 concentrations.
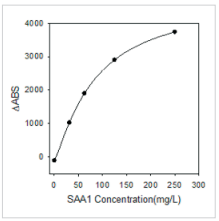
Fig.2 Calibration curve of SAA1 inparticle-enhanced turbidimetric immunoassay
Clinical Comparison
LFIA platform
28 samples from apparentlyhealthydonors and patients with acute orchronic inflammation were detected with the CUSAg LFIA SAA1assay. As shown in Fig.3, thedetection signals of T line wereproportional to the concentration of theSAA1in the sample tested bycommercial kit.
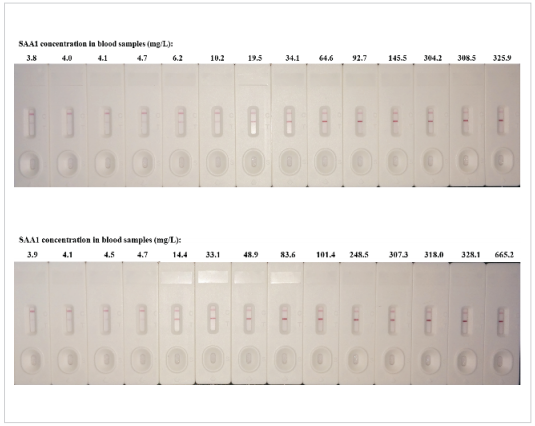
Fig.3 Semi-quantitativedetection of the concentration of theSAA1in the blood samples
LETIA platform
Anti-SAA1 monoclonal antibodies were also evaluated in medium-scale clinical tries with22blood samples from donates. Fig.4 showed that the correlation coefficient(r) is as high as 0.93between in-house latex reagents and commercialSAA1 immunoassay. These results show good agreement between the two systems.
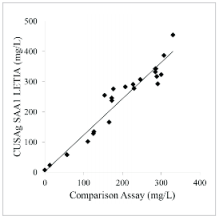
Fig.4 Clinicalcomparison of CUSAg LETIA immunoassay andcommercial immunoassays
A certain amount of excellent SAA1 protein (Catalog Number CSB-DP118B) is also offered, it could be used ascalibrator in immunoassay.
References
1.Gabay C, Kushner I (Feb 1999). "Acute-phase proteins and other systemic responses to inflammation". The New England Journal of Medicine. 340 (6): 448–54.
2.Tape C, Tan R, Nesheim M, Kisilevsky R (Sep 1988). "Direct evidence for circulating apoSAA as the precursor of tissue AA amyloid deposits". Scandinavian Journal of Immunology. 28 (3): 317–24.
Kisilevsky R, Manley PN (Mar 2012). "Acute-phase serum amyloid A: perspectives on its physiological and pathological roles". Amyloid. 19 (1): 5–14.
3.King VL, Thompson J, Tannock LR (Aug 2011). "Serum amyloid A in atherosclerosis". Current Opinion in Lipidology.
4.Malle E, Sodin-Semrl S, Kovacevic A (Jan 2009). "Serum amyloid A: an acute-phase protein involved in tumour pathogenesis". Cellular and Molecular Life Sciences. 66 (1): 9–26.

