Patelet-activating factor acetylhydrolase(Lp-PLA2)
Views:2093 Add time:2019-05-31 11:20:59
Since the beginning of 2015, CUSAg becomes the first Chinese manufacturer to introduce its Lp-PLA2 antibody pairs to IVD market. Much to our delight, they have been well-recognized by the domestic and International clients. Most of them apply our Lp-PLA2 antibodies to large scale IVD assay reagents production. We have always put Lp-PLA2 project to our top priority and have never ceased the pursuit of being the global No.1.
On March 2018, an article titled Measurement of Lipoprotein-Associated Phospholipase A2 by Use of 3 Different Methods: Exploration of Discordance between ELISA and Activity Assays published in AACC Clinical Chemistry Magazine, which attracted the attention to global clinical laboratory market.
Lp-PLA2 enzyme activity test method is more consistent with gold standard LC-MS/MS. Being the first FDA approved Lp-PLA2 assay, correlation of Diadexus’s ELISA-measured mass concentration and the enzymatic activity concentration is just 0.39. Only serum sample processed by denaturant and separated lipoprotein from Lp-PLA2, the binding site will be exposed. After measuring by ELISA, mass concentration will be consistent with the enzymatic activity concentration.
Sample processing involves dissociation time, temperature, efficiency and stability after dissociation, which brings difficulties to the industrial production of diagnostic reagents. There is limit for the direct use of enzymatic reagent range. It can only be used for large biochemical analyzers, immunochromatography and chemiluminescent platforms are not available.
In order to meet the requirement of diagnostic accuracy and adapt to a variety of detection platforms, CUSAg scientific team have spent lots of efforts in screening new antibodies. Eventually, they have successfully developed antibodies which can evade the lipoprotein binding sites without denaturing agents. It is consistent with the results of the enzymatic detection of the well-known diagnostic reagent manufacturer DiaSys Diagnostics Systems GmbH. Additionally, three antibody pairs suitable for latex turbidity, chemiluminescence and immunofluorescence chromatography applications bring great benefits to customers.

Lp-PLA2 is an independent risk marker for atherosclerotic plaque rupture and thrombotic events. It is an accurate risk assessment marker for patients at high risk of acute atherothrombotic recurrence. Unanimous recommendation from authoritative guides at home and abroad.
Lp-PLA2 Guidelines& Recommendations
· 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults recommends that Lp-PLA2 test for moderate-risk asymptomatic adults may be considered to further assess cardiovascular events Risk (IIb).
· 2013 ACC/AHA Guidelines for Cardiovascular Risk Assessment recommends that new markers be considered for patients who are asymptomatic primary prevention and who are still unable to determine whether treatment is needed after a risk assessment.
· 2011 AHA/American Stroke Association Guidelines for Primary Prevention of Stroke recommends that inflammatory markers such as hs-CRP or Lp-PLA2 can be identified to high-risk stroke patients (IIb / B).
· European Society of Cardiology 2012 Cardiovascular Disease Prevention Clinical Practice Guidelines recommends that patients with high-risk recurrence of acute atherothrombotic events can be tested for Lp-PLA2 to further assess the risk of recurrence (IIb / B).

CUSAg offers a series of excellent mouse anti-human Lp-PLA2 monoclonal antibodies. Linearity, clinical sample evaluation have been tested on the company’s latex turbidimetry immunoassay, chemiluminescent immunoassay and immunofluorescence platforms. The result shows that it has high correlation with Lp-PLA2 diagnostic kit by continuous monitoring method from DiaSys Diagnostic Systems GmbH. These indicate that CUSAg Lp-PLA2 monoclonal antibodies can meet the requirement on different applications.
A.Latex Turbidimetry Platform
CSB-DA113BmN⑧ and CSB-DA113BmN⑨ have been mixed together at 1:1 ratio and conjugated to latex micro beads to make preparation for latex turbidimetry reagents. 40 serum samples with assigned value from the enzyme test kits of DiaSys Diagnostic Systems GmbH. The two groups of reagents test result has a correlative greater than 0.95 compared with enzymatic assay. It indicates that CUSAg antibodies can be used on latex turbidimetry immunoassay application.
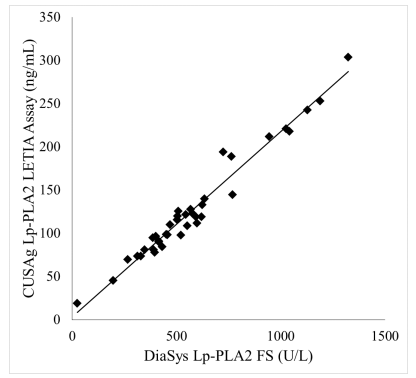


Figure 1: Comparison test of serum samples between latex turbidimetry and enzymatic assay
B. Chemiluminescence Immunoassay Platform
CSB-DA113BmN⑦ acts as a capture antibody and conjugated to Magnetic bead microsphere. CSB-DA113BmN② acts as detection antibody conjugated with HRP, which establish chemiluminescent immunoassay system.72 serum sample(dilution factor is 5) assigned with enzyme test kits have been tested. Sample requires no dissociation. The result shows in Figure 2. It indicates that CUSAg antibody test result is correlated with enzyme diagnostic test kits. The correlation coefficient is greater than 0.9. It can meet the requirement of chemiluminescent immunoassay development.
Buffer is this system is 1% Triton X-100, 0.25% sodium deoxycholate, and 20 mmol/L Tris pH 8.0
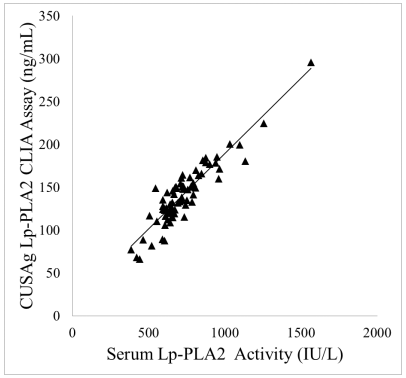


Figure 2: Comparison test of serum samples between chemiluminescent immunoassay and enzymatic assay
C.Immunofluorescence Chromatography Platform
CSB-DA113BmN③ acts as capture antibody stripped to NC memberane, CSB-DA113BmN⑤ acts as detection antibody which sets Lp-PLA2 Immunofluorescence Chromatography Platform. 45 serum samples (dilution factor is 50) have been quantitative tested, test result is in Figure 3. It indicates that the company's self-manufactured immunofluorescence test strips are highly correlated with the results of the enzymatic method and can be used in immunochromatography platforms.
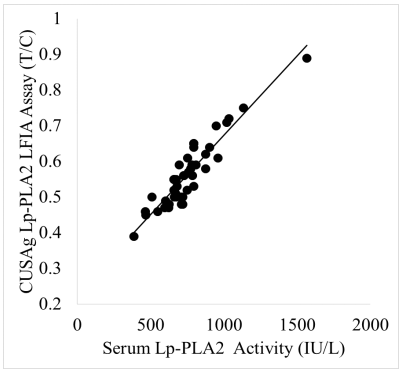


Figure 3: Comparison test of serum samples between immunofluorescence chromatography assay and enzymatic assay

